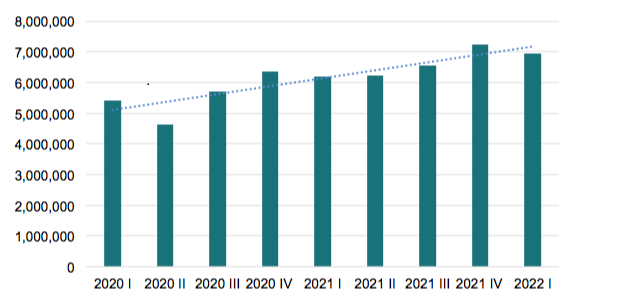
In Q1 2022, 26.6 million transactions were made in LZVS, of which 6.2 million were package decommissioning and dispensing. This is 12% more than in the same period last year, according to the Latvian Medicines Verification Organization (LZVO) data.
Since the launch of the verification system in Latvia, the number of packages verified and decommissioned has been gradually increasing each year. This is mainly due to two reasons: firstly, the number of end-users gradually increased during the first years of the system’s operation, as well as the experience and quality of using the system improved. The second reason is the growth of the market and the increase in the consumption of medicines. According to the State Agency of Medicines of Latvia, the turnover of medicines in community pharmacies increased by 2.1% in 2021 compared to 2020.
Number of dispensed packs in LZVS per quarters
Unique identifier on each medicine package guarantees safety of the medicine. Manufacturer put the code on package, and it is multiple times online verified on the way to the consumer. At the end of the supply chain – in a pharmacy or healthcare institution – the unique code is decommissioned from the system before the medicine has been checked out or used. This procedure ensures that the same identifier can’t be used, preventing falsified medicines from entering the legal supply chain.
If the system does not recognize the unique identifier during the verification, an alert has been generated, which is received and investigated by the Health Inspectorate. According to information at the disposal of LZVO, all alerts in Latvia have been of technical origin and not alerts due to falsified medicines. In March 2022 the share of alerts affecting end-users of LZVS was on average only 0.01%, which is in line with the EMVO target of 0.05%
The establishment of the medicines verification system is stipulated by the EU Falsified Medicinal Products Directive (2011/62/EU) and a delegated regulation stipulating detailed rules on the safety features of medicinal products for human use (EU 2016/161). This system works in 29 European countries since 9 February 2019. All prescription medicines and one over-the-counter medicine – omeprazole – are checked by the system.
