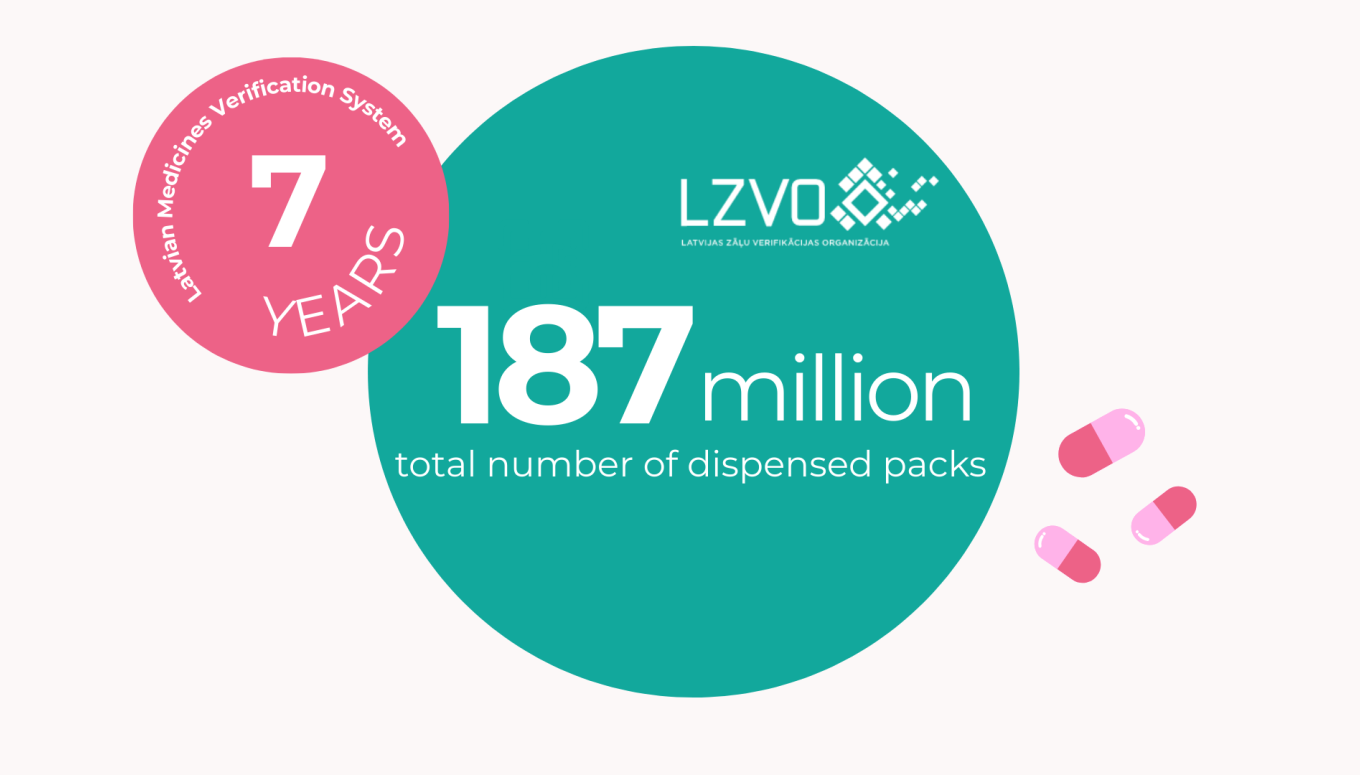
09.02.2026

News
20.01.2026
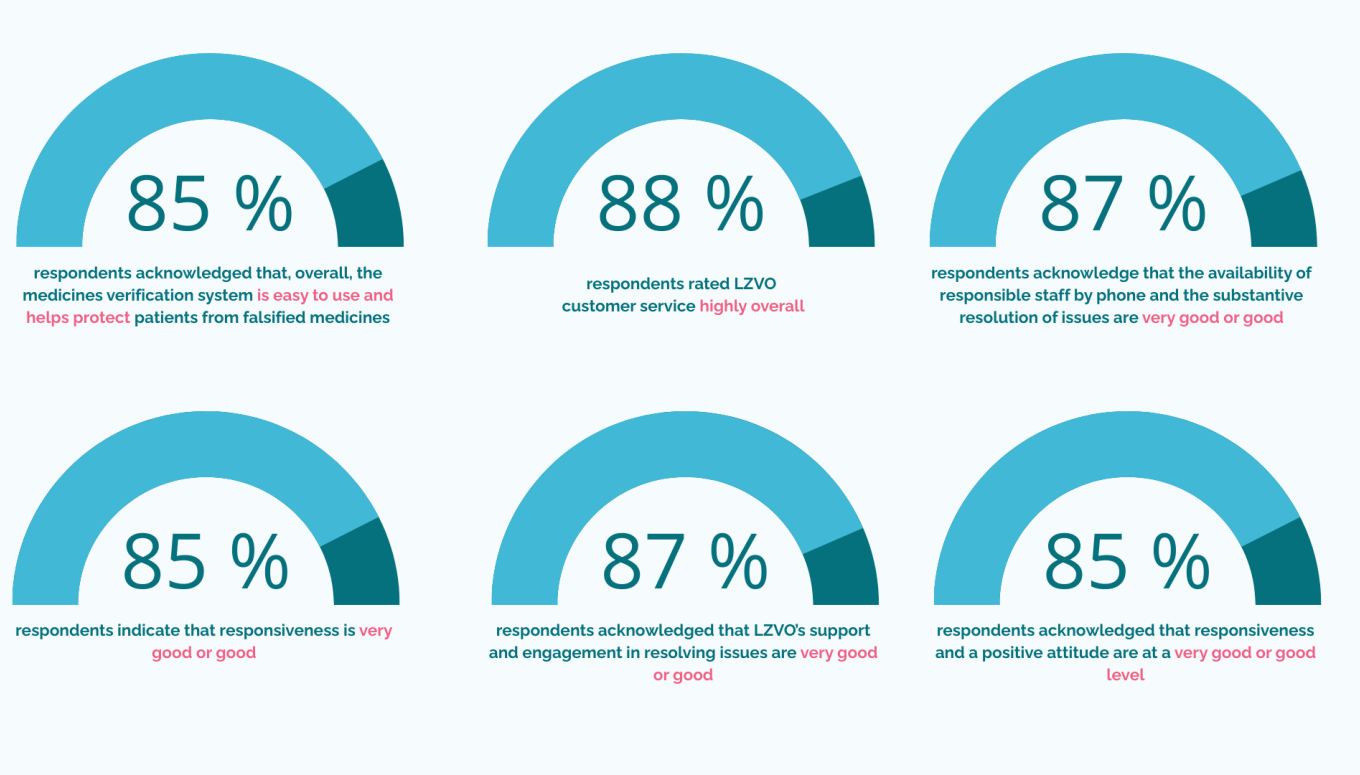
LZVO receives high customer service ratings for the third year in a row
20.01.2026
Thanking SWS for the high customer service rating
23.12.2025
Happy holidays!
08.12.2025
LZVO Newsletter No. 15. December 2025
24.11.2025