Verification System
The new medicines verification system was introduced in Europe on 9 February 2019. Its purpose is to prevent falsified medicines infiltrating the legal supply chain and ultimately from reaching European patients. The system is based on a European Union regulation (Directive and Regulation).
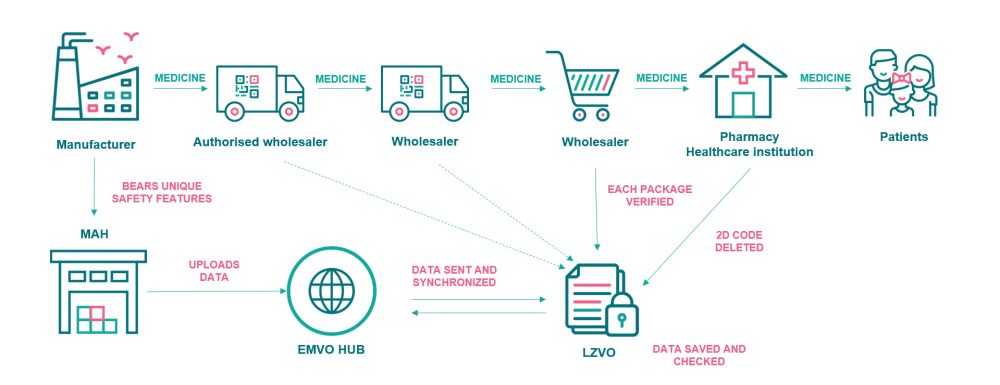
The verification system applies to the prescription medicines and omeprazole (an over-the-counter medicine). During the manufacturing of medicines, each packaging is labelled with a special 2D barcode containing unique identifiers and anti-tamper device. Embedded in this barcode will be a product code, serial number, batch number and expiry. Pharmaceutical manufacturers send details of the information in the barcode to the Latvian Medicines Verification System (LZVS) via the EU Hub. European Medicines Verification Organisation (EMVO) manages the central ‘European Hub’ that connects all the national and supranational data repositories in Europe and serves as the platform to allow the authenticity of medicines anywhere in the supply chain in the European Economic Area (EEA) to be verified.
Since the medicines can pass through several warehouses of distributors and wholesalers on their way to patients, the wholesalers are obliged to verify the authenticity of medicines bearing safety features on a risk approached basis or in specific circumstances as set out in the Commission Delegated Regulation (EU) 2016/161. Pharmacies, hospitals and other healthcare institutions must verify the authenticity of medicines and decommission them from the LZVS before they are supplied to patients. The unique 2D code should be verified and decommissioned in a pharmacy right before dispensing the medicine to the patient. A healthcare institution can decommission the medicine at any time, while it possesses it. The unique code allows to verify the name of the medicine, its dose form and strength, the size and type of the packaging, the lot number, the expiration date and other information. It confirms the authenticity of a medicine.
All medicines with a false identity (packaging, labelling, name, content, strength), origin (manufacturer, country of manufacturing, country of origin, marketing authorisation holder) and history (manufacturing documents and documents related to the employed networks of distribution) are considered as falsified.
The Latvian Medicines Verification System (LZVS) is a part of the common European verification system that has a central database or a repository and links together ~30 national or supranational systems. LZVS or the national repository was created and is maintained by the Latvian Medicines Verification Organisation (LZVO) that is connected to the European database online and ensures verification of medicines in pharmacies and healthcare institutions 24/7/365.
The establishment and maintenance of the national verification system in Latvia (LZVS) is funded by the marketing authorisation holders (MAHs) who market in-scope products in Latvia and authorised parallel importers. MAHs must onboard to the EU Hub which is managed by EMVO and upload unique identifier data that has been placed on packs to the EU Hub. The data then is transferred from the EU Hub to the national repository for the market where the packs are intended to be sold.
The pharmaceutical wholesalers, pharmacies, hospitals and other healthcare institutions are obliged to onboard to LZVS and use the system for verification of medicines.
The medicines verification system is a very extensive, but, at the same time, very fast European level database that stores information about the safety features of all medicinal products and allows to identify medicines and verify their authenticity.
LZVO signed a contract with German company Arvato Systems GmbH (Arvato) in February 2018 to develop, set up and maintain the Latvian medicines verification system.
Arvato has also developed national verification systems of several other EU member states.