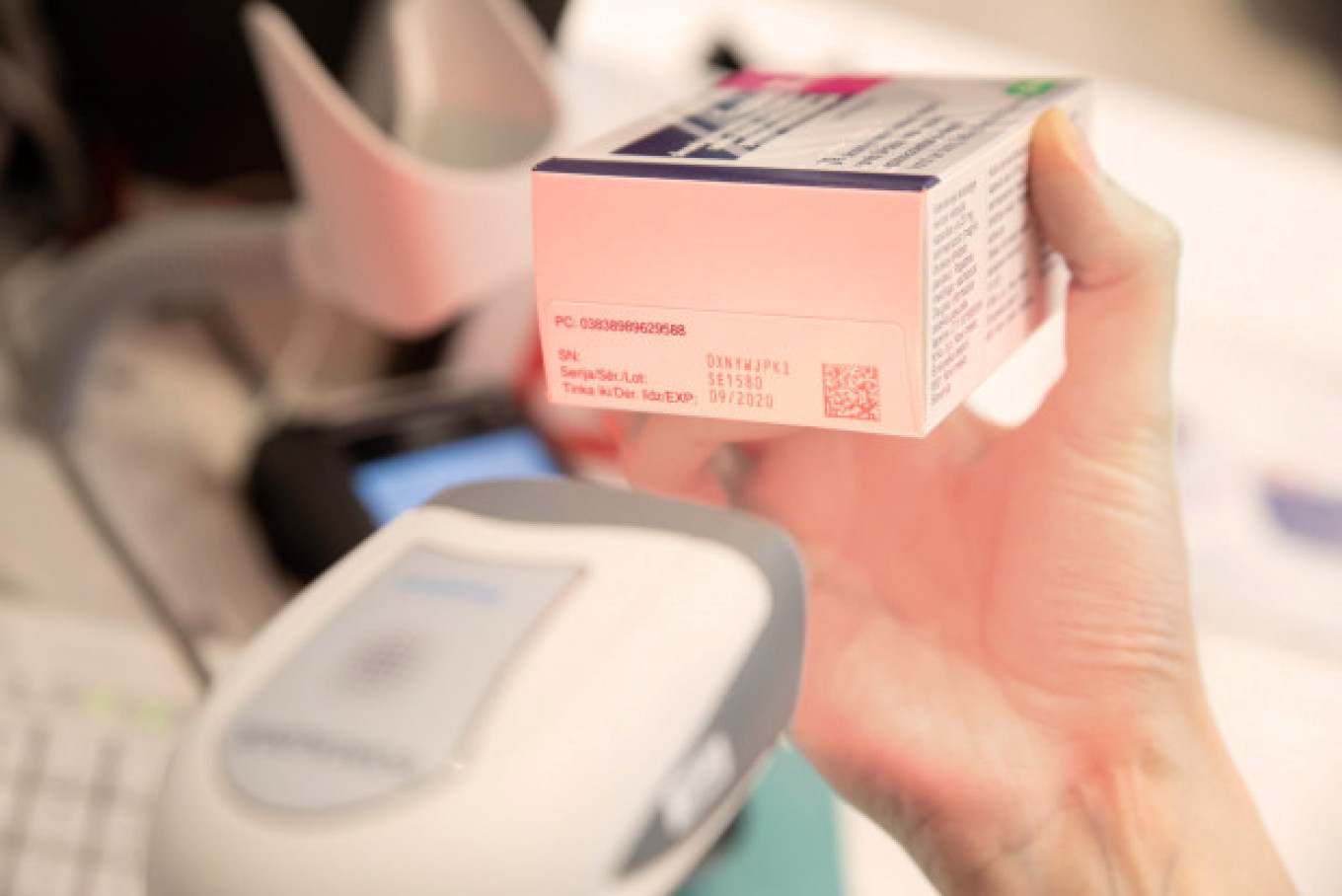
A new medicines safety testing system has been successfully implemented in Latvia for five months, which has been simultaneously introduced in more than 30 European countries with the aim to prevent the risk of patients receiving or buying falsified medicines.
Since 9 February when the system started operating, end users in Latvia – pharmacies and healthcare institutions – have already verified and decommissioned from the system more than 3 million medicine packages.
The basic principle of the verification system operation is that each medicine package is labelled with a unique code in the factory during the manufacturing process, and the data of all producers and product codes are uploaded and stored in a common European database. This code is checked at all stages of medicine distribution on the way to the consumer. The unique code is deleted from the European Verification System before selling the medicine at a pharmacy or at any moment in hospital, outpatient facility, medical centre, medical practice or dental clinic. Thus, it will be no longer possible to sell the second medicine package with the same code, e. g., copied onto a falsified package – the system will display a warning and the medicine will be withdrawn from circulation and investigated.