Kristina von Sydow, General Manager, e-VIS

The Swedish e-verification system in numbers
- 1 000 000 transactions per day
- 110 million packs per year in the scope of FMD
- More than 1 500 locations connected
- Alert rate varies between 0,02% to 0,04%, in total number around 400-500 alerts per day.
- Decommission rate close to 100% (also confirmed by the Swedish NCA when comparing actual dispensing numbers with deactivation numbers in the SMVS)
- Number of falsifications found through the system: 0
Success factors for the implementation of FMD in pharmacies in Sweden
Five years has passed since Sweden and the rest of Europe implemented FMD. Almost all prescriptions in Sweden are electronic since many years. The pharmacies in Sweden have since a long time been connected to national databases and been dependant on complex IT-systems in their dispensing process. Another important prerequisite for implementation of FMD and electronic prescription is that Sweden has had a well-functioning national product register where end-users can retrieve product data, such as product codes and if the product must have safety features or not. Thanks to this matureness when it comes to IT- implementation and use of IT in the dispensing process the implementation in Sweden went relatively easy. The pharmacies in Sweden were used to scan packs in the dispensing process since this was already a part of the so called “picking control”. The pharmacy scans the code carrier on the pack to fetch the product code and can match that with the product chosen for dispense. Implementing FMD has therefore not added any extra step in the standard dispensing process. Another key success factor is having a QMS, fundamental to pharmacy operations. Even though the dispensing process did not have to change much to implement FMD, many processes had to be updated and reviewed with respect to FMD. Except the delegated regulation, national legislation has demands on pharmacy operation, some examples are:
- Keep a record of all dispensing orders with a reference to the precisions used.
- Record the unique identifiers in the record of dispensing orders so that a specific pack can be related to a specific customer.
From stabilisation period to full implementation
Sweden decided to start FMD with an implementation period. There where however no exceptions for the pharmacy to not control and verify every pack. All packs with safety features should be scanned by pharmacies from day one. The risk of errors in the data upload however, lead to the decision that pharmacies could ignore warnings regarding packs that where not found in EMVS. The pharmacy could dispense packs that was not found in EMVS without decommissioning them.
The stabilisation period only applied to pharmacies. Wholesalers and MAHs was from day one required to follow the regulation. Also, there was never a stabilisation period for packs generating alerts and warnings related to the pack status. These packs had to be withheld from dispensing and the alerts had to be investigated from the start.
This approach gave e-VIS and the supply chain the possibility to monitor which kind of problems that caused alerts without affecting the supply chain. The stabilisation period was ended in a step wise approach so that errors and problems could be fixed in a controlled manner.
Another key to the success of implementing FMD in Sweden is the pragmatic collaboration between all actors in the supply chain and as well the Swedish NCA. There is a clear direction for all that the EMVS is in place to provide a safer supply of medicines.
Alert management and communication
The pharmacies have procedures for how to handle and interpret warning from EMVS and how to quarantine packs. There are many types of responses from the EMVS, and the pharmacy procedures and IT-systems have been updated so they support the pharmacist in correct interpretation of the EMVS response.
When a pharmacy has ruled out that the alert in the SMVS is caused by their own handling, they need to report the impacted pack to the MAH and e-VIS. This is done by using the already existing web-based complaints channel in Sweden. Through this channel the pharmacy has a direct link to the MAH that immediately can start to investigate or ask e-VIS for support. Since a few years back e-VIS has a national Alert management system (AMS) in place where pharmacies and wholesales easily can document root causes for alerts, and e-VIS can monitor that all alerts has been investigated. Sweden will also in the future connect the national AMS to the European AMS-hub.
Improving the recall process in Sweden and the Nordic countries
In 2023 the Nordic NMVOs published a common Best Practice on how to use the EMVS when recalling a batch or withdrawing a product from the Nordic market. The recall process through the EMVS is immediate and irreversible, and therefore requires correct handling of the system, but for those MAHs that have these processes in place the EMVS provides for an extra safety layer. If a batch needs to be recalled, the EMVS can prevent the packs from being dispensed to patients without any delay. (Please see our Nordic Best Practice for more information.)
What can be achieved by using the system?
- Falsified packs can be identified and stopped.
- It prevents authentic packs that leaves the legal supply chain from returning to saleable stock.
- It prevents packs belonging to a recalled batch or a withdrawn product are being handed out to the patient. It immediately stops further distribution in the supply chain of these packs.
- It gives assurance to pharmacies, healthcare and wholesalers that pack at hand is manufactured by the MAH.
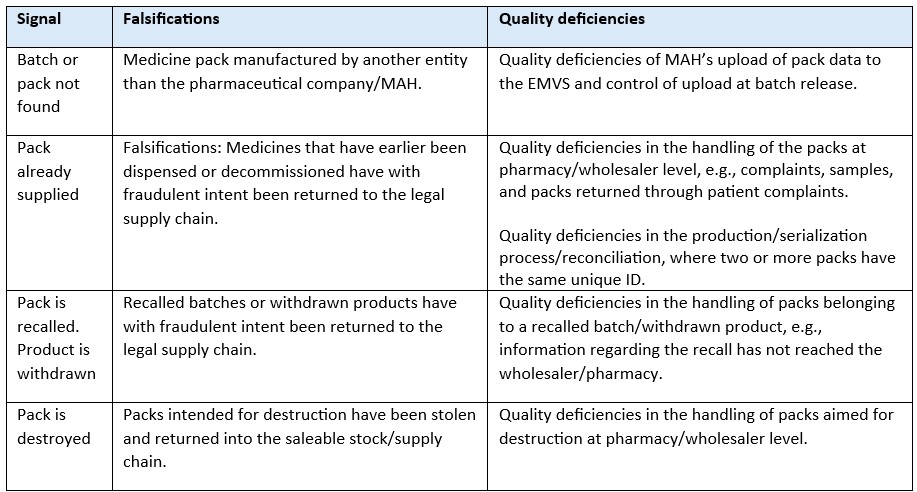
Table: The system can both identify falsifications and quality deficiencies.
The difference between a falsified medicine and a quality deficiency is the intention. For the future of the EMVS we foresee a higher understanding of how the system in addition to the ‘core purpose’ be used to simplify the supply chain and increase quality. The example of using the EMVS to recall batches swiftly and safe is a practical example.
To respond to the question in the header, can we have more confidence in the medicines supply chain after FMD has been implemented? Yes. The implementation of FMD prevents falsifications to reach the legal supply chain and in the rare case of a fake coming into the legal supply chain the system will identify that pack. The system improves the recall process and identifies quality issues. And this is just the beginning of safeguarding the legal supply chain.
Resent knowledge from both Sweden and Europe highlights the fact that most falsifications reach patients through illegal sale. The supply chain actors in Sweden are doing their best to protect the legal routes, but to fully protect patients from the harm of falsified medicines we also need to see more actions on the illegal sale.
