The European Medicines Verification System, operating since 2019 in accordance with the EU Directive and Delegated Regulation, is a comprehensive, pan-European system covering all EU and EEA countries, connecting 2,800 pharmaceutical companies, 4,000 wholesalers, 110,000 pharmacies and 6,000 hospital pharmacies online, including all Latvian end users. Altogether a total of 175 people are working in 29 national organisations and the European Medicines Verification Organisation.
Number of EMVS transactions
The European Medicines Verification System (EMVS) comprises a central EU Hub managed by the European Medicines Verification Organisation (EMVO) and national medicines verification systems in 29 countries across Europe. The EMVS is utilized by all registration authorization holders launching medicinal packages into the EU market. In principle, every FMD pack of prescription medicine in Europe must be equipped with the unique identifier that is uploaded through the EU hub to the national verification systems. EMVS is used by all wholesalers in the EU legal medicine supply chain to check packs in certain situations. EMVS is used by all pharmacies and hospitals in Europe for verifying and decommissioning prescription medicines. Disruptions in the operation of EMVS could adversely affect the supply of legal medicines in Europe, posing a risk to patient access to safe products. Therefore, continuous operation, improvement, security, and monitoring of the system are essential. The functioning of the EMVS as an ecosystem depends on all participating systems and organizations.
In the first half of 2023, the availability of the EMVS was above 99.9%. The average response time is significantly below the 300-millisecond threshold (< 31 ms), including inter-market (IMT) and high-volume transactions. In the first half of 2023, the number of transactions conducted by EMVS end users continued to grow, reaching 3.4 billion transactions per quarter, which is a 21% increase compared to the same period in 2022. Less than 1% of transactions were inter-market (IMT).
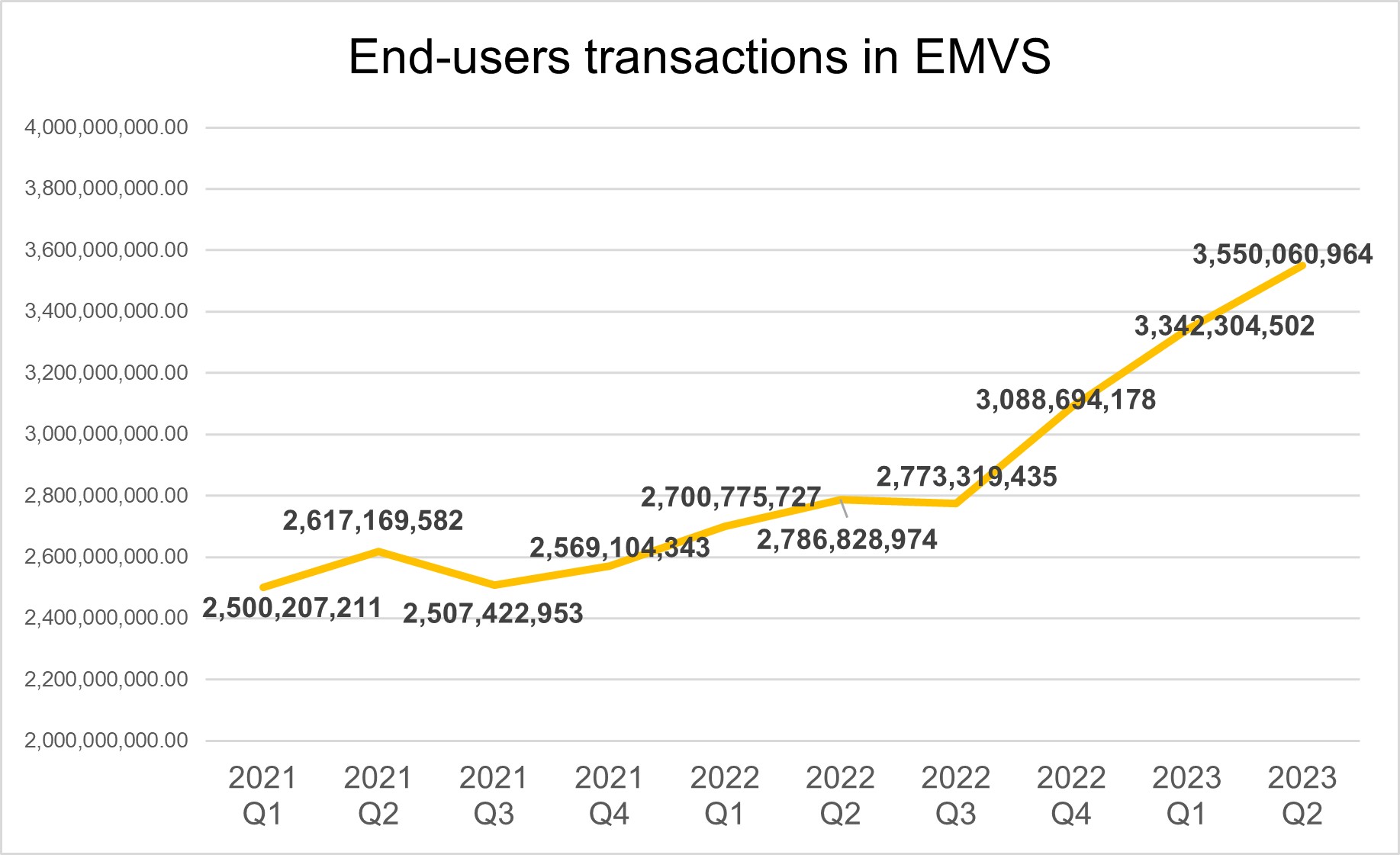
In the first half of 2023, the number of transactions increased by 21% compared to 2022, reaching an average of 3.4 billion transactions per quarter.
The number of uploaded packages has typically remained stable:
- 11.3 billion in 2020;
- 10.3 billion in 2021;
- 10.9 billion in 2022;
- 5.8 billion in the first half of 2023.
In the first half of 2023, 14% of the total number of uploaded packages were intended for several markets (multimarket).
Stabilisation periods in European countries
Some of the larger European countries, which initially opted for rather lengthy stabilisation periods that seemed user-friendly at first, are now grappling with the uncertainties caused by unclear regulations and shifted deadlines. Therefore, Latvia's approach of operating without a stabilisation period has been very effective and intentional. National competent authorities, such as the Health Inspectorate, provided significant support in the initial stages of system implementation by initially advising system participants on connecting to the system, without opting for the most drastic approach.
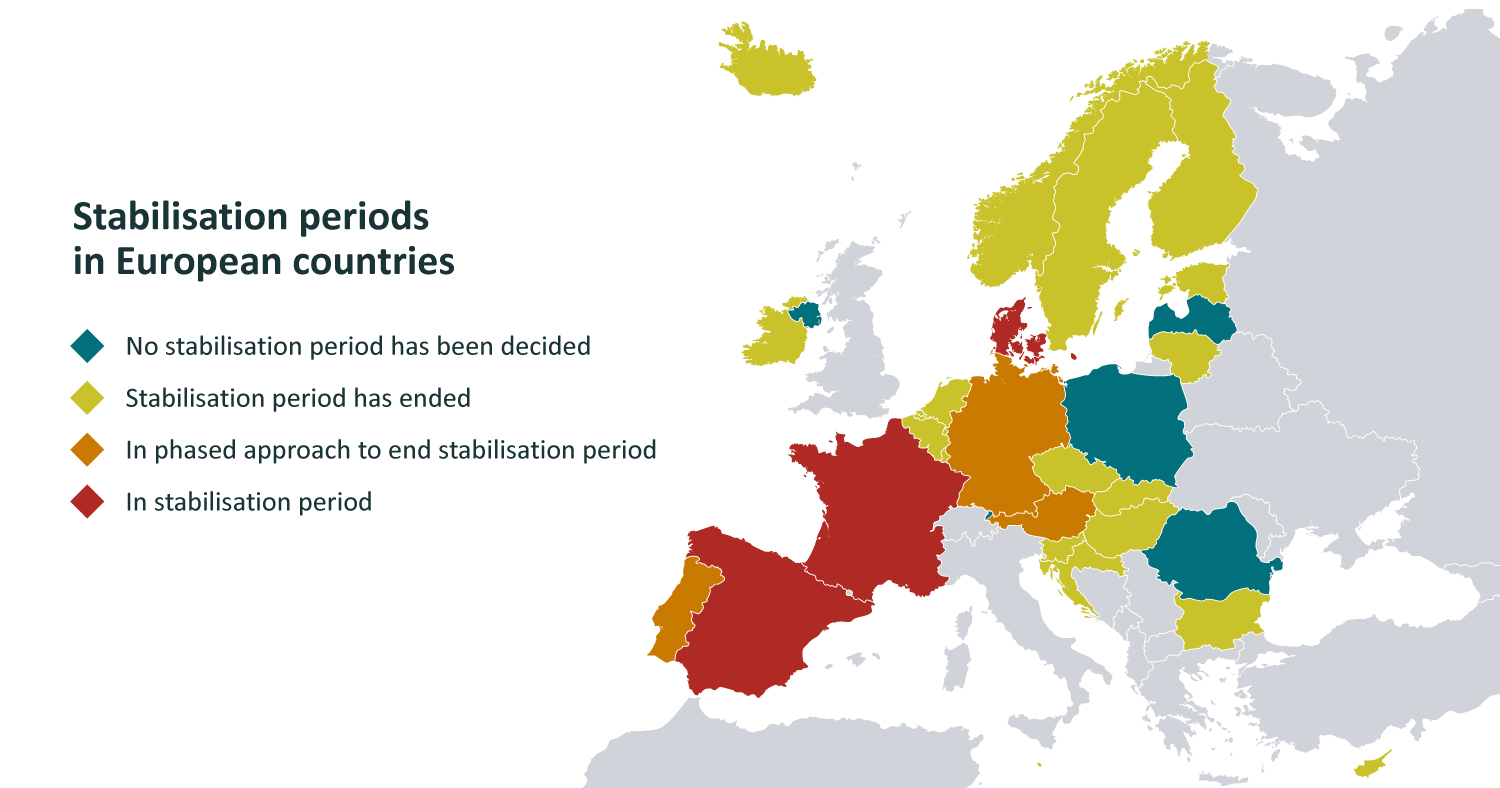
Data: EMVO, January 2024
Alerts
In the initial stages of the system’s operation, end-users were most concerned and had many questions about alerts. Both initially and currently, the majority of alerts are related to technical or procedural errors.
The proportion of alerts in Latvia is significantly below the target set by the European Medicines Verification Organization (0.05%) – this is a very high efficiency indicator, as in many countries using the system, the proportion of alerts remains significantly higher.
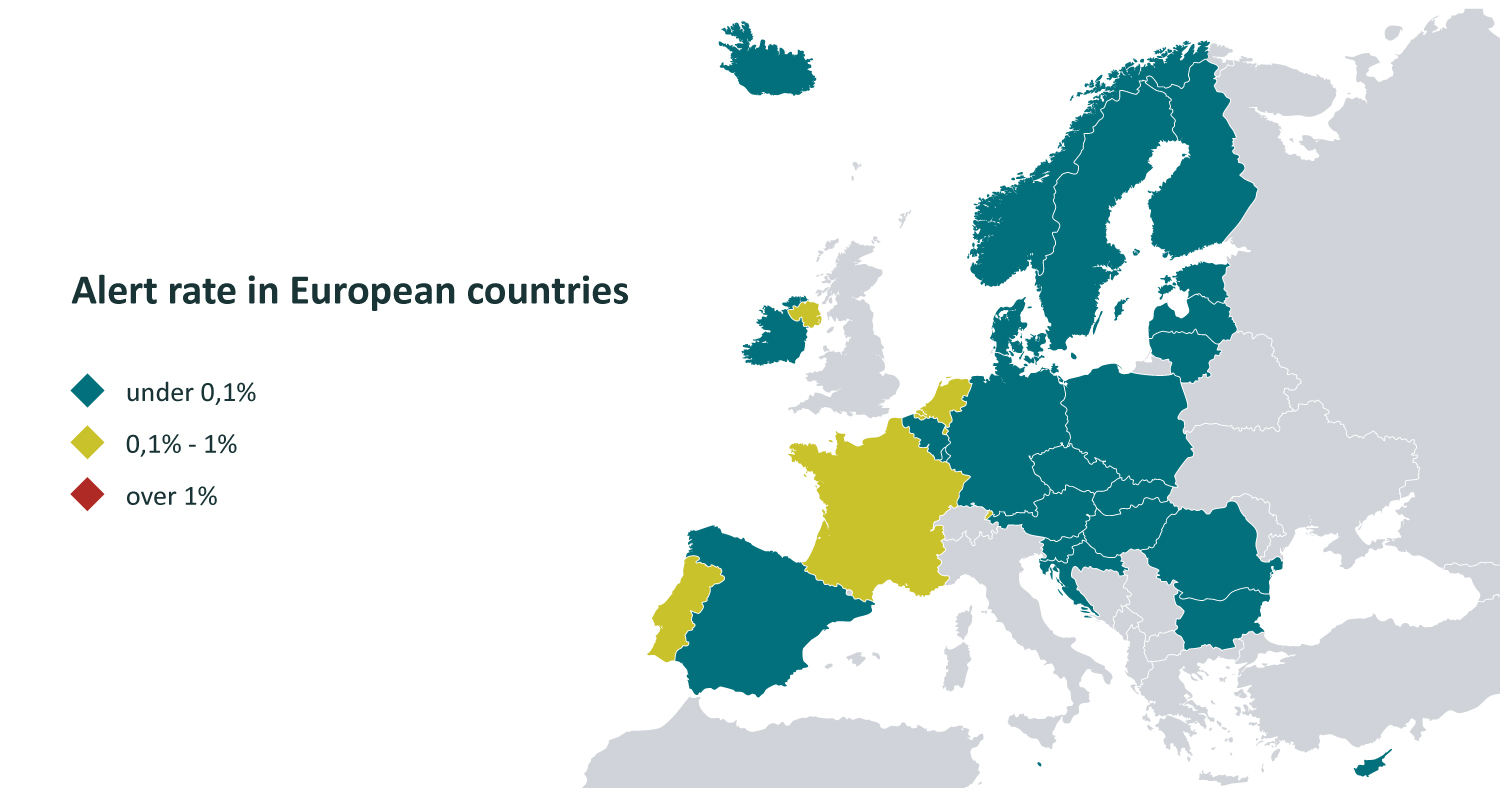
Data: EMVO, January 2024
