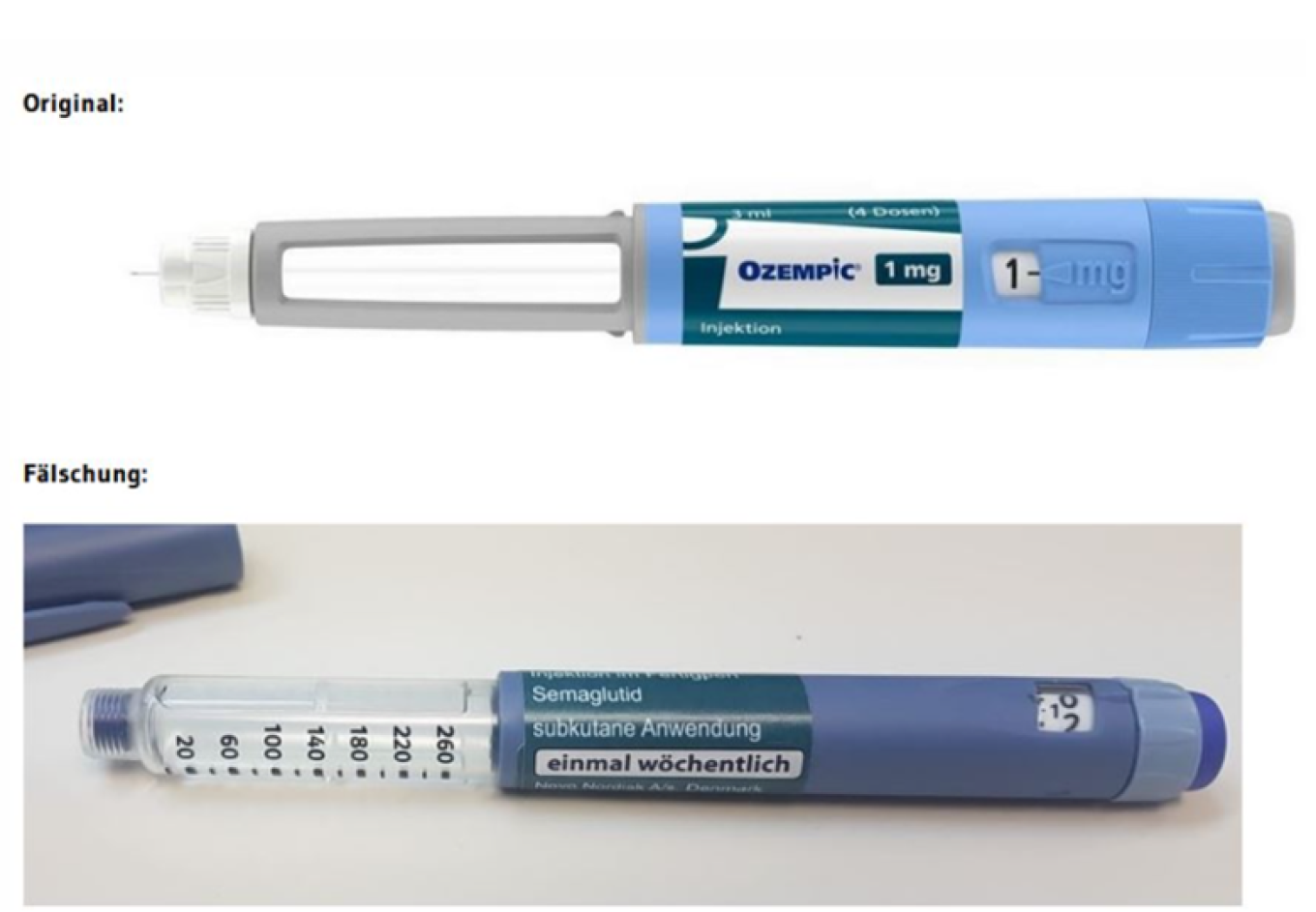
The German Federal Institute for Medicines and Medical Devices (BfArM) has confirmed that the medicines verification system in Germany has detected a counterfeit versions of medicine Ozempic®, which were promptly removed from circulation and has not reached the patients. Responsible authorities in Germany, as well as the manufacturer of the original medicine, Novo Nordisk, are investigating this case.
Ozempic® is a prescription medicines, used for the treatment of diabetes in adults. Ozempic® is an injectable solution administered with a pen.
According to information available to the Latvian Medicines Verification Organization (LZVO), there is no Ozempic® from this specific batch with counterfeit issues in Latvia.
For safety reasons, a specific batch of these drugs was also recalled in Poland.