Mari Asser, REKS Processes and Quality Manager
In Estonia, the organisation for the verification of the authenticity of medicinal products is called REKS and the verification of packaging is therefore called "reksimine". Estonia's NMVO (REKS) is a small-scale organisation, with 2 people and a so-called "robot worker", or REK-IS, to manage alerts. The local robot allows certain alerts to be solved 24/7 and so the REKS employee does not have to intervene at all.
The system is well accepted and implemented by all end users and by the Estonian NCA. The following is a brief overview of the different ways in which packaging is checked in Estonia and the way in which alerts are managed.
Pharmacy
Estonia has two pharmacy software. In both of them, authenticity verification is integrated into the normal workflow, i.e. it is carried out together with the digital prescription and is linked to the sales process when the packaging is sold by 2D code.
If an alert occurs, the pharmacy has the option of either forwarding the alert information via the REK-IS system to the REKS for investigation/processing or, if the pharmacy already knows why the alert occurred, it can stop the sale and issue the pack.
The REK-IS processing system receives the alert information directly from the pharmacy information system. The Estonian Medicines Agency considers all warnings received through the REK-IS procedure as documented (including the e-mail receipt) and the pharmacy does not need to document anything further. REK-IS works 24/7 and certain procedures are automatic, for example:
- Exception check - returns an immediate response to the user when an exception is detected and the user can issue the package. The REK-IS has a list of batches known to raise an alert during the check (including unauthorised but 2D coded medicines, analogue coded medicines, expired products, etc.). The list of exceptions is managed by REKS and the process for adding data to it is agreed with the Estonian Medicines Agency. In the case of an automated procedure for exception checking, the pharmacist does not need to reply to REKS and can immediately dispense the pack.
- Russian keyboard control (symbols in codes) - if the REK-IS system detects the presence of symbols in GTIN or SN codes, REK-IS returns an automatic response that the alert is not actionable. The user does not need to reply anything further.
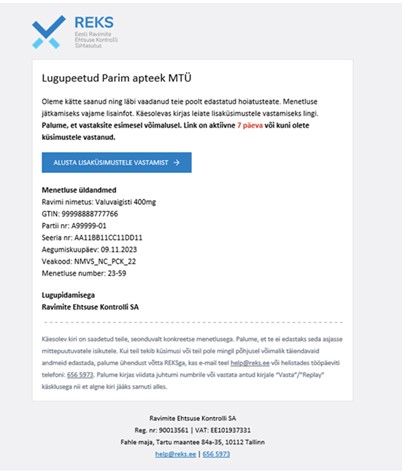
- Expiry date check - if the REK-IS system detects that the expiry date has passed, an automated message with a link to reply is sent to the sender. The user forwards the reply via the link. Depending on the response, the system may either close the procedure automatically or it may be forwarded to the manual procedure where the REKS staff will be contacted again if necessary.
How the procedure works
- All automated procedures start immediately with a link to a reply letter 24/7.
- Notification of the start of manual procedures must be sent separately by ordinary e-mail. The REKS will initiate the procedure and will in most cases forward additional questions via a link for reply. In 99% of cases, REKS will ask for a photo to accompany the request if it was not originally sent. The closure of the procedure is always indicated by a separate e-mail.
- The reply link will be active for a certain time., during which time the recipient will have the opportunity to reply to the questions. The system sends two reminders before the link expires.
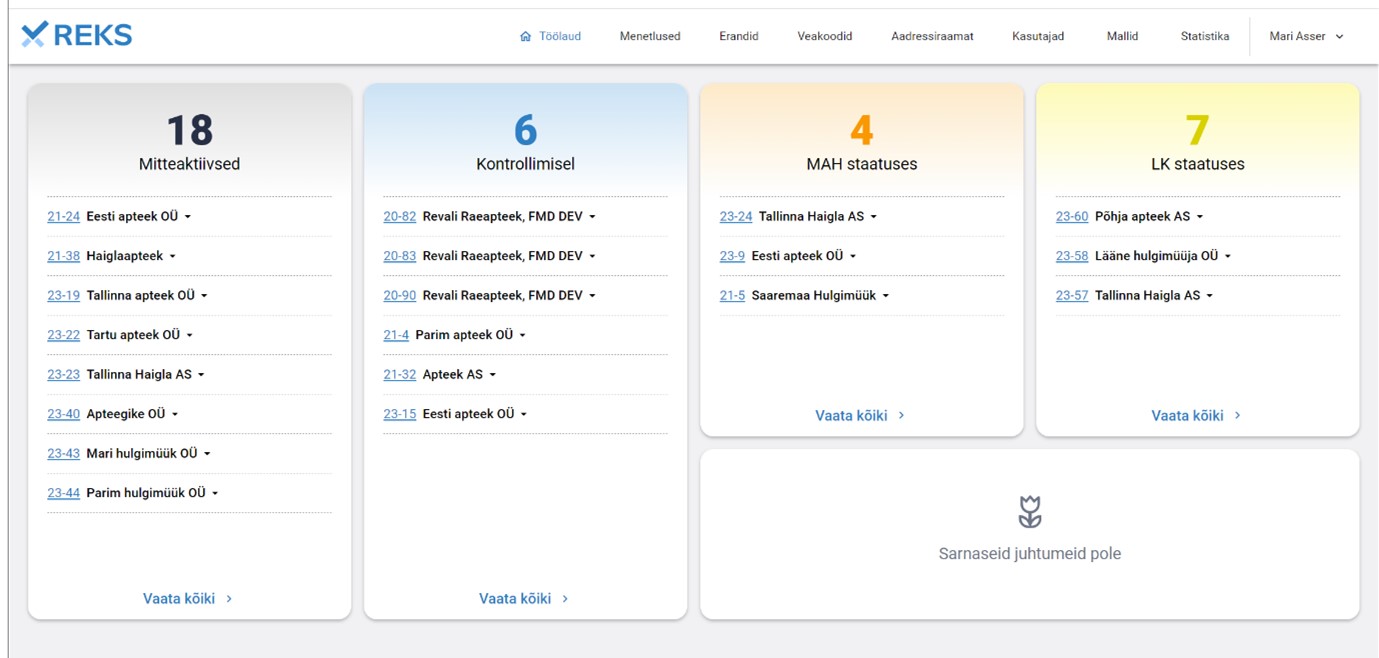
Example of the REK-IS dashboard
Hospital Pharmacies
Hospital pharmacies carry out checks on the authenticity of packaging in accordance with the Regulation, i.e. at the time of receipt. If a warning appears, hospital pharmacies will check whether the exception is already known and can be found on the list. If not, the hospitals transmit the information on the basis of the corresponding form to the REK-IS procedure system and REKS checks the packaging information.
Most of the time, unauthorised medicines end up on the list of exemptions. Although there is no obligation to check them, it has become common practice for pharmacies to ask REKS for confirmation that the batch is in order and to prefer that unauthorised medicines are also included in the list of exceptions.
Alerts are also processed in the REK-IS system for hospital pharmacies.
Wholesale
Wholesalers have based their checks on the fact that unauthorised packages received from a designated wholesaler are generally not fully checked. However, spot checks are carried out and the REKS is also proactively informed of those batches which are not authorised and for which the details have not been uploaded to our system, but which give rise to a warning when the authenticity of the packaging is checked. Such batches are entered by REKS on the list of exceptions on the basis of a notification from the wholesaler.
Like hospital pharmacies, wholesalers also use the REK-IS online form to send notifications. However, there are also those who send information to REKS by regular e-mail.
Marketing Authorisation Holder / Producers
REKS has informed manufacturers/authorisation holders that, if necessary, we will contact them ourselves on a case-by-case basis regarding the alert. REKS does not need to proactively communicate information as a statistical overview. ´
Most of the communication with the MAH is by e-mail. The new REK-IS solution allows for the possibility to send out response e-mails in English via a link, which significantly facilitates the processing of warnings, both in terms of content and time.
Catching marketing authorisation holders/manufacturers whose medicines are unauthorised in Estonia has proved to be the most difficult. There have been situations where it has taken several weeks to process an alert because the MAH/manufacturer has not replied to an email, or it has been difficult to find the right company responsible for a particular product.
Supervisory Authority
In Estonia, the Estonian Medicines Agency oversees the entire system of packaging authenticity checks. The inspectorate has previously identified minor problems where packs have been dispensed despite an alert and the pharmacy has no evidence to prove this. In the course of the monitoring, the Agency has not identified any end-user who has ignored the obligation to check the authenticity of the packaging.
REKS has a very close and good cooperation with the Medicines Agency. It has included the verification of the authenticity of the packaging as part of its routine monitoring. In addition, the Agency assists the REKS in the handling of certain alerts.
