February 9, 2024, will mark 5 years since operations of a medicine verification system began across Europe and Latvia. The system’s purpose is to ensure that all people receive only safe medicines, eliminating the possibility of counterfeit drugs reaching patients through legal supply chains.
Over time, the number of checked and decommissioned medicine packages in the Latvian Medicine Verification System (Latvijas zāļu verifikācijas sistēma – LZVS) has gradually increased, the proportion of alerts has decreased, and the LZVS end user registration system has been fully sorted.
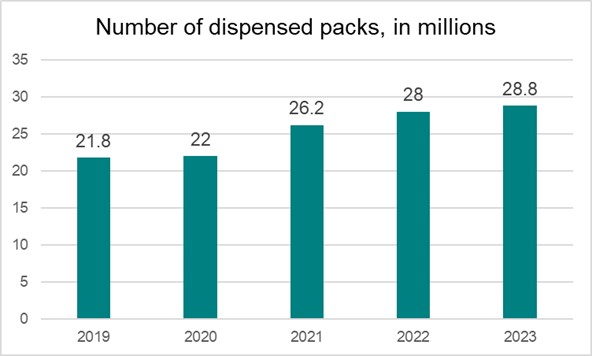
Number of verified packages
Since the launch of the verification system in Latvia, the number of checked and decommissioned medicine packages has been gradually increasing each year. This is primarily due to two reasons – firstly, the first few years of the system’s operation saw a gradual increase in the number of end users and an improved system use experience and quality. The second reason is the growth of the market and an increase in medicine consumption.
In 2023, a total of 107.8 million transactions were carried out in the LZVS, of which 28.8 million correspond to the decommissioning of medicine packages in Latvia as packages were supplied to citizens. This represents a 3% increase compared to 2022.
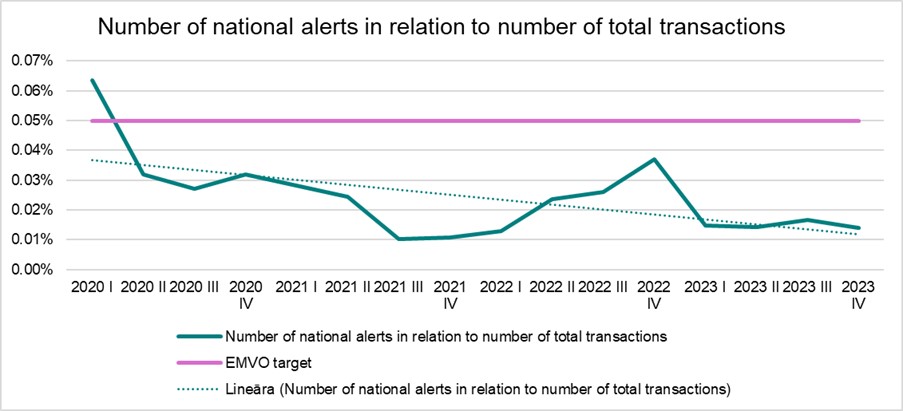
Alerts
In 2023, the proportion of alerts affecting end users in Latvia was only 0.01% on average – significantly less than in the early stages of the system's operation, when the proportion of alerts made up around 0.2% of the total number of transactions.
Both in the early stages of the system’s operation and now, the majority of alerts have been related to technical or procedural errors. Alerts demonstrate that the system is operating effectively and are useful due to their ability to signal both data loading errors on the manufacturer’s side and end-user errors when dispensing medications.
The proportion of alerts in Latvia is significantly below the target set by the European Medicines Verification Organisation (0.05%) – this is a very high efficiency indicator, as the proportion of alerts remains significantly higher in many system countries.
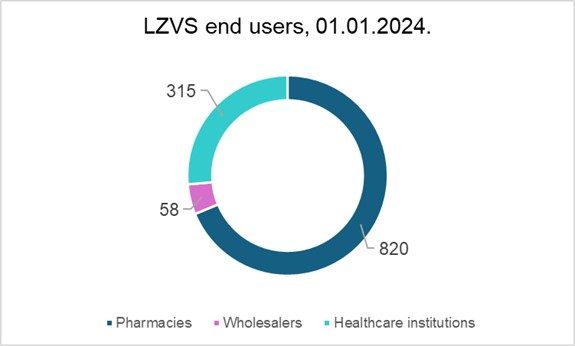
System end users
At the start of 2024, the total number of LZVS end users was 1193, comprising 820 pharmacies, 58 wholesalers, and 315 medical institutions.
Upon its launch in 2019, the system had already attracted 1043 users – pharmacies and medical institutions – in Latvia. In the first few years of the system’s operation, the number of users gradually increased each month, leading to 100% of end users in Latvia, mandated to join the system by regulatory acts, having done so at present.
The number of end users of the system constantly experiences some minor fluctuation, as only legitimate users are allowed access. For example, medical practices whose authorization to purchase medicines has expired are excluded from the system.