09.02.2026

News
24.11.2025
The digital infrastructure of the LZVS will be migrated to a cloud environment
11.11.2025
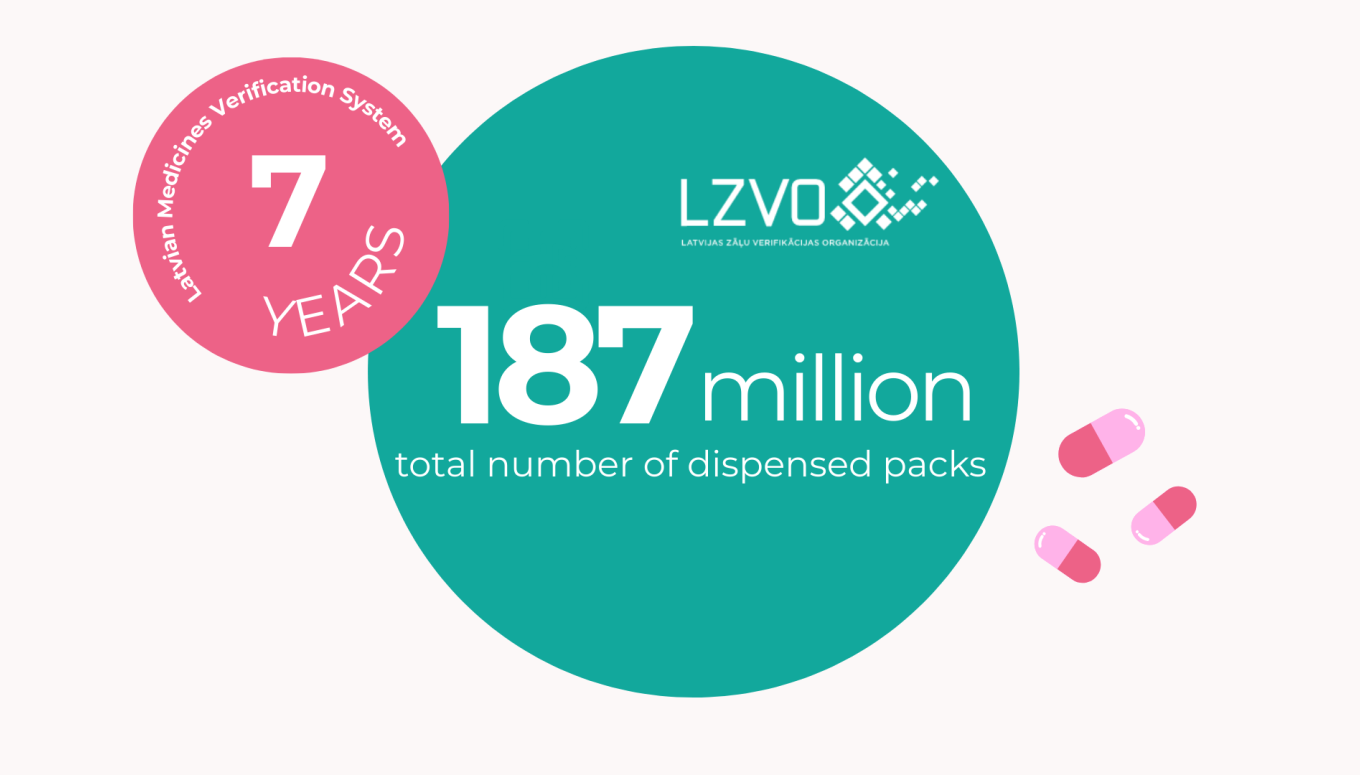
Almost 25 million prescription drug packs dispensed in Latvia this year
23.10.2025
EMVO launches its renewed website
02.10.2025
LZVO warns: Rapid increase in online advertising and sale of counterfeit weight-loss medicines
10.09.2025